Berkelium is an element found in the periodic table that has the symbol “Bk” and atomic number 97. This element is a member of two element series, namely actinide and transuranium, and it is also the fifth transuranium element discovered by scientists after neptunium, plutonium, curium, and americium. Because there was only one gram of berkelium that was produced in the United States since it was able to be synthetically created in 1967, berkelium is considered one of the rarest elements on Earth. To know more about how berkelium was synthetically produced, let us take a closer look at the interesting discovery of berkelium.
Discovery of Berkelium
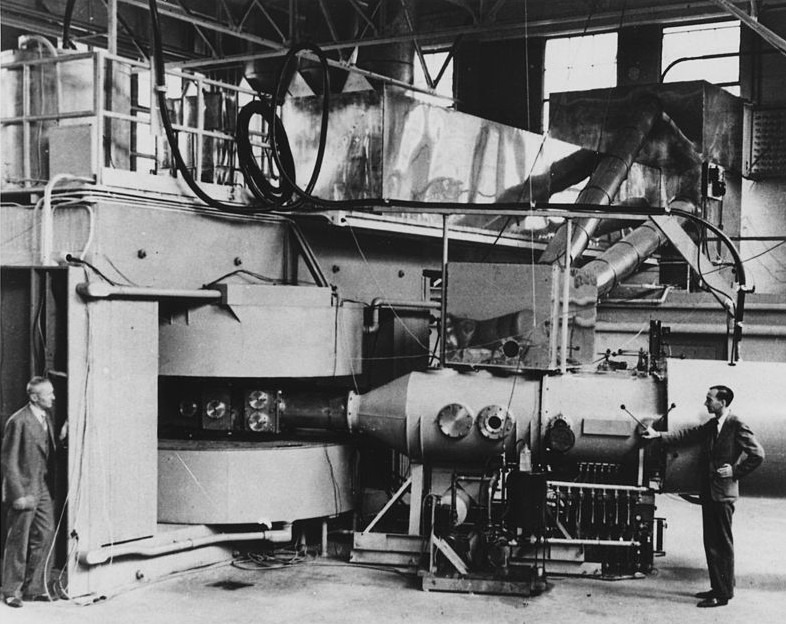
Scientists believed that before the official discovery of berkelium, some agencies were already able to produce the element when they began experimenting on nuclear weapons and machines. However, berkelium was first intentionally synthesized in December 1949 by chemists Stanley G. Thompson, Alberto Ghiorso, Kenneth Street Jr., and Glenn T. Seaborg, who have also isolated and identified the element in the same year. These four chemists were able to synthesize berkelium using the 60-inch cyclotron, a type of particle accelerator that is built and located at the University of California, Berkeley. Interestingly, the cyclotron was also utilized five years prior to the discovery of element 95 (americium) and element 96 (curium), and the discovery was also conducted by the same chemists.
The name “berkelium” came from the tradition of the four chemists to name their new discoveries after the United States and the University of California, Berkeley. Americium is named after America and serves as the analogue to europium, while curium is supposed to pay homage to Pierre and Marie Curie, who were the pioneers in the research for radioactivity. To keep the tradition, they named element 97 “berkelium,” which is inspired by the name of the university where they were working. Along with berkelium, the four chemists also discovered element 98, californium, around the same year, and that element is named after California. In a discovery report published by the chemists, they stated that another reason why they named element 97 as “berkelium” as its chemical homologue, terbium (element 65), is named after the town of Ytterby, Sweden, so it feels right to name the new element after a location where it was discovered. However, californium’s name is not inspired by its lanthanide analogue dysprosium (element 66), but from the state of California.
What made berkelium hard to synthesize for the chemists was that it was difficult to separate from its final products, and a sufficient amount of americium, another rare element, should also be present in the synthesis. To synthesize berkelium, the scientists would first coat the right quantities of americium nitrate solution with platinum foil, then it will be evaporated, and the residue would be converted by utilizing americium dioxide. After that process, there would also be several more steps before successfully synthesizing a small amount of berkelium, which is the reason why many chemists would opt not to synthesize it at all because of the difficulty of producing it without taking more than 20 steps. Chemists calculated that you would need more than 200 days to properly synthesize a sufficient amount of berkelium that is fit for scientific research.
In addition, there were only a few amounts of berkelium that were produced over the years because chemists are unable to find uses for the element, although it is crucial in the research for other elements. The largest amount of berkelium ever produced was 22 milligrams, which was synthetically produced in more than 300 days, with 250 days being the irradiation process and 90 days being the purification process. The 22 milligrams of berkelium was produced in Oak Ridge, Tennessee, in 2009. With the help of berkelium, the chemists at Oak Ridge, along with the Joint Institute for Nuclear Research in Dubna, Russia, were able to discover the first six atoms of element 117, tennessine, but they were only able to find out about it after bombarding berkelium with calcium-48 ions for approximately 150 days.
Another reason why not there is not a lot of berkelium produced since the 60s was that it is believed to be toxic for humans, although as of 2019, there were no reported side effects of berkelium to the body. However, there are some experts that speculate that a dangerous amount of berkelium in the body can promote the growth of cancer cells and the damage of red blood cells. Furthermore, some stated that berkelium could affect the skeletal system more than any other system in the human body. Despite the assumed danger of berkelium, the element is still being synthetically produced in numerous labs in modern times, as scientists believe that they may one day find benefits in producing and utilizing the element for the greater good.