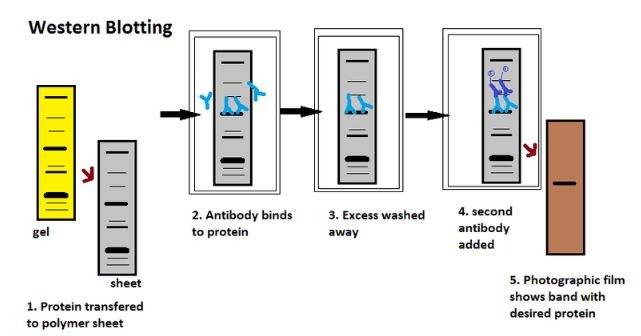
A detailed insight into each stage of the western blotting process.
Western blotting is an analytical technique that researchers use to detect specific proteins in complex protein samples. The technique enables researchers to separate proteins by a variety of factors, label a protein of interest with an antibody, and then examine this protein. Western blotting has paved the way for impressive advances in scientific and medical research, which the life sciences journal BioTechniques documents in its print issues and on its multimedia website.
Here, BioTechniques delves into the electrophoresis process that researchers perform to separate proteins in a sample, explains the five steps they then follow to complete the western blot, and details the control measures they can implement to generate accurate results.
SDS-Polyacrylamide Gel Electrophoresis
Before beginning the western blot process, researchers separate the proteins in the sample, often through SDS-polyacrylamide gel electrophoresis. This process involves applying an electric charge to separate proteins according to their electrophoretic mobility, which depends on the structure of the proteins and their charge and molecule size. The high resolution of the gel electrophoresis and the strong specificity and sensitivity of the immunoassay make it possible for researchers to detect target proteins as low as 1ng.
Polyacrylamide gel (PAG) is a 3D mesh networks polymer made up of a cross-linker (methylene bisacrylamide) and acrylamide under the catalyzation of ammonium persulfate. PAG offers various electrophoretically desirable features that make it versatile. Not only is it synthetic, chemically relatively inert, and thermo-stable, but it is also strong and transparent, and researchers can prepare it with an array of average pore sizes.
SDS: Separating Proteins by Size
SDS creates an electrostatic repulsion that causes proteins to unfold into a rod-like shape. This eliminates differences in shape as a factor for separation in the gel. Therefore, the electrophoretic mobility of the SDS-protein subunit compound is based on molecular weight, not protein size or charge.
The researcher mixes the sample with SDS and heats the mixed sample to at least 60°C to encourage protein denaturation and depolymerization. This helps the SDS bind and enables both the negative charge adherence and the rod-shaped formation. The researcher may add a bromophenol blue dye to the sample so they can track progress during the electrophoretic run. They also add glycerol to increase density and accelerate the migration of the sample.
The “Laemmli” System
The researcher applies a buffer system with different pH values during the gel electrophoresis process. For example, they might use the tris-glycine or “Laemmli” system, which stacks at a pH of 6.8 and resolves at a pH of approximately 8.3-9.0. However, these pH values can encourage disulfide bond formation among cysteine residues in the proteins. This is because the reducing agent in the loading buffer doesn’t co-migrate with the proteins and because the pKa of cysteine ranges from 8.0-9.0.
That said, new developments in buffering technologies can overcome this challenge by resolving the proteins at a pH below the pKa of cysteine. New technologies also include reducing agents that uphold a reducing environment in the gel. Using buffers with low pH values makes the acrylamide gel more stable, allowing laboratory teams to store it for long periods before use.
How Voltage Affects Electrophoresis
When the researcher applies voltage to the sample, the anions and negatively charged proteins migrate toward the positive electrode in the lower chamber. The trailing ion is Gly¯, and the leading ion is CI¯. SDS-protein particles don’t migrate freely between the Gly¯ of the cathode buffer and the Cl¯ of the gel buffer. The voltage drop between these buffers causes the proteins to compress into a micrometer-thin layer-stacking gel layer.
In this gel layer, the proteins that have more negative charges per unit migrate quicker than those that have fewer negative charges per unit. So, proteins with a lower molecular weight migrate faster. The boundary moves through a pore gradient, and the protein stack disperses gradually because of the frictional resistance increase in the gel matrix.
Choosing the Right Gel
Researchers use polyacrylamide gel electrophoresis (PAGE) to separate proteins that range in size from 5-2,000 kDA. They can control the pore size provided by the polyacrylamide gel by controlling the concentrations of acrylamide and bis-acrylamide powder in the gel.
The researcher usually pours a stacking gel (5%) on top of a resolving gel (5%, 7%, 10%, 12%, or 15%) and gel comb (which forms the wells and defines the lanes for the proteins, sample buffers, and ladders). Researchers select the concentration of the resolving gel based on the size of the protein they want to identify or probe. The smaller the known weight, the higher the concentration they should use.
The Five Stages of Western Blotting
Having separated the proteins in the sample, researchers then follow five steps to complete the western blotting process. These steps are: transfer, blocking, primary antibody incubation, secondary antibody incubation, and protein detection and analysis.
1. Transfer
The researcher transfers (or blots) the separated proteins from the gel onto a matrix. This is usually a polyvinylidene difluoride (PVDF) or nitrocellulose (NC) membrane. PVDF membranes are ideal for detecting proteins with small molecular weights because of their high resolution, sensitivity, and affinity. Researchers need to sink PVDF membranes in methanol before use to activate positive charge groups in the membrane.
Meanwhile, NC membranes produce little non-specific staining. While they’re easy to use and cost-effective, they’re also fragile, and it’s easy to erase small molecular proteins while washing. Researchers should apply NC membranes with different pores depending on the transferred proteins’ molecular weight. The smaller the pore of the membrane, the tighter the combination between the membrane and the proteins should be. NC membranes of 0.45 µm and 0.2 µm are most common: 0.45 µm is ideal for proteins that have molecular weights over 20 KD, and 0.2 µm is ideal for proteins that come in below 20 KD.
Wet Transfer Versus Semi-Dry Transfer
The most common transfer methods for proteins are wet transfer and semi-dry transfer. Wet transfer involves suspending the Gel-Membrane-Filter sandwich in the transfer buffer. The electrode plate produces a high-intensity electric field that encourages the proteins to transfer from the gel to the membrane. On the other hand, semi-dry transfer involves placing the Gel-Membrane-Filter sandwich between filters loaded with transfer buffer.
2. Blocking
Having transferred the proteins to a membrane, the researcher must then block unreacted sites on the membrane to minimize the non-specific binding of proteins. They can achieve this using blocking buffers like non-ionic detergents or inert proteins.
The most common blocking buffers are non-fat dry milk, BSA, gelatin, casein, and Tween-20. Each offers unique benefits. For example, BSA is ideal for blots where a phosphorylated protein is the target, and casein is ideal for blots that use an alkaline phosphatase-conjugated secondary antibody. Meanwhile, although non-fat dry milk is typically the most cost-effective option, researchers should avoid using this as a blocking reagent for blots that use a biotin-conjugated antibody because milk contains biotin and glycoprotein.
3. Primary Antibody Incubation
The researcher then stains the proteins with a primary antibody that binds to the target protein. The researcher should select a primary antibody based on the antigen they are trying to detect and validate this antibody before use. Once finished, they should wash off any unbound antibody.
Polyclonal and monoclonal antibodies work well in western blotting. Polyclonal antibodies recognize more epitopes than monoclonal antibodies and tend to have higher affinity. Although they destroy a few epitopes, blot results should be stable. Meanwhile, monoclonal antibodies recognize single specific antigenic epitopes. This means they tend to have higher specificity and lower background. If the target epitope is destroyed, this will impact the blot results.
4. Secondary Antibody Incubation
After rinsing off any unbound primary antibody, the researcher’s membrane is exposed to a specific enzyme-conjugated secondary antibody. This secondary antibody binds to the primary antibody, which will have reacted with the target protein.
The most common secondary antibodies are anti-rabbit and anti-mouse immune globulin. This is because the most common host species for primary antibodies are rabbits and mice. Some researchers may also use goat anti-rabbit and goat anti-mouse immune globulin as secondary antibodies. Researchers should select their secondary antibody based on the species of animal in which the primary antibody was raised. For example, if the primary antibody is a rabbit polyclonal antibody, the secondary antibody should be an anti-rabbit antibody.
5. Protein Detection and Analysis
When a substrate reacts with the enzyme that is bound to the secondary antibody, protein bands appear on the membrane. Two of the enzymes utilized most often are horseradish peroxidase (HRP) and alkaline phosphatase (AP). The researcher can then evaluate the target protein levels through densitometry and by examining the location of the protein bands.
Following color development, the researcher can imprint the pattern of the separated proteins onto film or capture this pattern with a western blot gel imager. They can compare the band position to the protein ladder to estimate the size of the target protein.
Five Western Blotting Controls
Control is vital during the western blotting process. Good control design improves the likelihood of accurate and specific test results by making it easier to identify any problems quickly. These are the five most common controls seen in western blotting.
1. Positive control verifies the working efficiency of the antibodies with a lysate from a tissue sample or cell line that expresses the protein the researcher is trying to detect.
2. Negative control checks antibody specificity (so the researcher can identify non-specific binding or false positive results) using a lysate from a tissue sample or cell line that doesn’t express the protein the researcher is trying to detect.
3. Secondary antibody control checks secondary antibody specificity without adding the primary antibody to the membrane. This indicates any non-specific binding or false positive results caused by the secondary antibody.
4. Blank control checks the membrane nature and blocking effect by not adding the primary or secondary antibodies to the membrane.
5. Loading control checks the sample quality and the performance of the secondary antibody system.
Loading Controls
Loading controls are antibodies for proteins expressed at equivalent levels in most cells and tissues. Researchers use loading controls to check that they have evenly loaded the lanes in the gel with the sample. This is especially important when drawing comparisons between expression levels of a protein in different samples. Loading controls are also useful for checking that the gel has been evenly transferred. Where a transfer isn’t even, researchers can use the loading control bands to quantify the protein in each lane. It is essential to use loading controls in studies that will be published.
Western Blotting’s Growing Prevalence
The western blot has grown prevalent over the past 40 years, and researchers now use the technique in a wealth of applications. Today, researchers have cited the western blot over 5,000 times and have proven that the technique is essential to developments in research and diagnostics.
The peer-reviewed, open-access journal BioTechniques publishes the latest information on lab methodologies, techniques, and instrumentation. Lab researchers, other scientific professionals, and students around the world use the journal as a key resource to aid their practices. While the journal offers a host of insights and information, BioTechniques also uploads a plethora of webinars, videos, articles, podcasts, infographics, and eBooks to its website, where an online community engages in knowledge share and essential discussion.