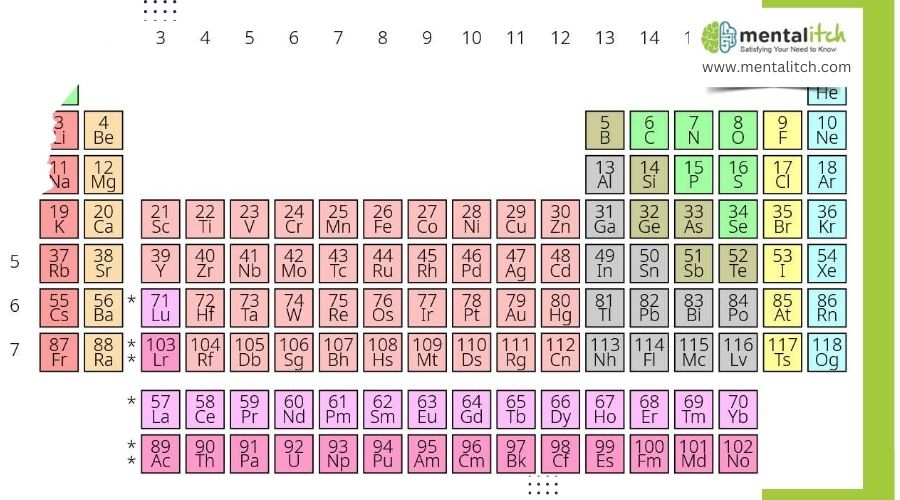
Of course, we all had encountered the periodic table during our chemistry classes way back then, but most of us remember only the more common elements such as oxygen, hydrogen, nitrogen, iron, gold, silver, mercury, calcium, and others. It’s been a long time since we studied them, and most of us don’t remember many of the unfamiliar and rare elements that usually occupy the bottom regions of the periodic table. These elements are very, very rare. Such elements are found in nature, synthetically made by man, or both.
Here, we focus on the rarest elements found on the periodic table and explain them one by one:
- Astatine
- Berkelium
- Protactinium
- Rhodium
- Osmium
- Iridium
- Oganesson
- Francium
- Technetium
- Neptunium
Astatine (At)
Astatine sits on the bottom row of the periodic table, with the symbol At and atomic number no. 85. It is the rarest naturally occurring element that is not a transuranic element. Transuranic elements are elements whose atomic number are greater than 92 — the atomic number of uranium. They are also unstable and prone to breaking down radioactively into other elements. When you look at the periodic table, you’ll find that astatine is also a member of the halogen family, belonging to Group 17 of the periodic table.
Besides being extremely rare, if you were to actually get a hold of astatine, it wouldn’t be around very long anyway. Astatine has a half-life of about eight hours. This means half of the Astatine would decay within 8 hours. And then, every eight hours, the other half of the astatine would disintegrate until, in a few days, it would be completely gone. Nada.
Now, you have an idea about how long (or short) an astatine exists. No wonder the name “astatine” derives from the Greek word astastos, which means “unstable.”
Astatine is very rare not only in the whole of the Earth but even in the entire universe. Scientists believe that no more than 25 grams of astatine can be found on the surface of the entire Earth.
Berkelium (Bk)
Another one of the rarest metals on Earth, berkelium, is a rare radioactive transuranic element with the symbol Bk and atomic number 97.
Berkelium is a synthetic element; it was first produced by scientists Stanley G. Thompson, Glenn T. Seaborg, Kenneth Street Jr., and Albert Ghiroso in 1949. At that time, they worked at the University of California in the city of Berkeley, where the element got its name. They bombarded an isotope of americium-241 with alpha particles using a special machine called a cyclotron. These experiments eventually led to the creation of berkelium-243 and two free neutrons.
Berkelium-247 is the element’s most stable isotope. It has a half-life of about 1,380 years and breaks down into americium-243 through alpha decay.
In 1962, the first visible amounts of berkelium compound — berkelium chloride — were first produced. They weighed about three billionths of a gram.
Since only small amounts of berkelium have been produced, this element is considered extremely rare. With the exception of its use in scientific research, this element has no known commercial usefulness.
Protactinium (Pa)
Protactinium is a chemical element with the symbol Pa and atomic number 91. It was first identified in 1913 by American chemist Kasimir (or Kazimierz) Fajans and German chemist Oswald Helmut Gohring. They named it brevium because of the brevity of the shortness of its half-life (about 1.17 minutes). In 1918 a more stable isotope version was independently discovered in 1917 (or 1918) by two groups of scientists — Otto Hahn (Germany)/Lise Meitner (Austria) and Frederick Soddy/John Cranston (both from Great Britain). It was then given the official name Protactinium. Another scientist, Aristid von Grosse from Germany, was the first to isolate naturally occurring protactinium in 1934.
Protactinium emits a silver-gray luster that can exist for some time in the air. It becomes superconductive below 1.4 K. It is one of the rarest and most expensive naturally occurring elements and is also quite toxic. It is found in uranium in very small quantities. In 1961, the United Kingdom Atomic Energy Authority (UKAEA) was able to produce 125 grams of 99.9% pure protactinium, although they had to process 60 tons of waste material in a 12-stage process — and at a cost of US $500,000.
Due to its rarity, terribly high cost, toxicity, and high radioactivity, protactinium is of very little use outside of scientific research.
Rhodium (Rh)
Rhodium is a chemical element with the symbol Rh and atomic number 45. It was first discovered by English chemist William Hyde Wollaston in 1803, shortly after his discovery of another element, palladium. He procured rhodium from a crude platinum ore, which was thought to have come from South America. From the sample, Wollaston removed the platinum and palladium, but from this procedure, he was left with a dark red powder, which turned out to be sodium rhodium chloride. Rhodium has the tendency to occur along with deposits of platinum. It is chiefly obtained as a byproduct of mining and refining platinum through nickel mining operations.
Rhodium is a noble metal, meaning that it is resistant to corrosion and oxidation (rusting) in moist air, unlike base metals (such as iron and tin). This makes rhodium a precious metal, just like gold and silver. But unlike the two, rhodium is very rare. In fact, rhodium can cost ten times as much as gold… or more! Despite this, rhodium is not feasible as a metal to make jewelry from because it is brittle and easily breaks, and due to its scarcity, it’s terribly expensive. However, rhodium is great for jewelry plating because of its shiny, mirror-like reflectivity that makes jewelry and shiner brighter through electroplating.
Besides its use in jewelry, rhodium is also used to make electrical contacts and in catalytic converters. However, it’s used more often as an alloying agent in other materials, such as palladium and platinum. These alloys are used to manufacture a lot of things, such as furnace coils, laboratory crucibles, and electrodes for spark plugs in aircraft.
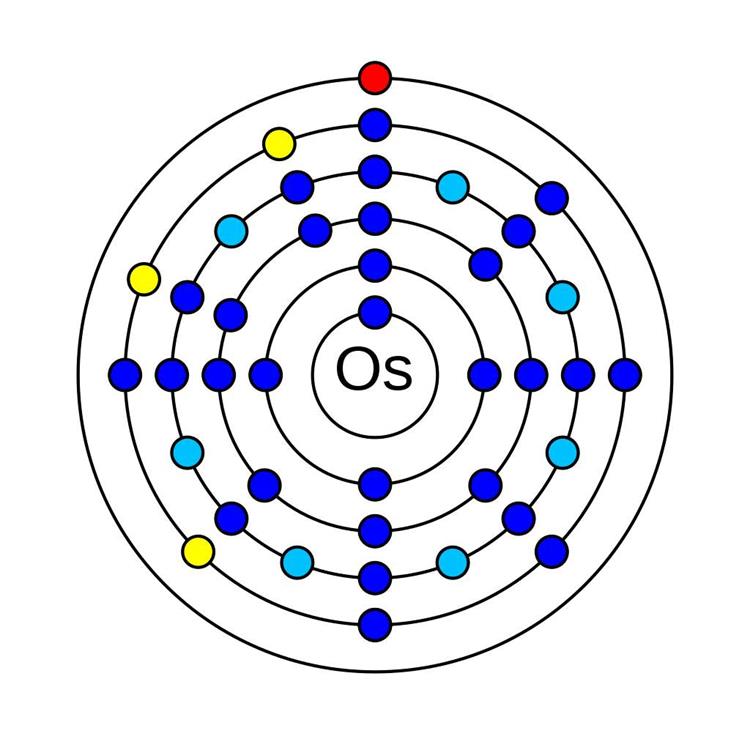
Osmium (Os)
Osmium is another very rare element with the symbol Os and atomic number 76. in the periodic table. Osmium is hard, brittle, silvery, and has a blue cast. It is a transition metal that belongs to the platinum group and is found as a trace element in alloys, mostly in platinum ores.
In 1803, osmium (together with iridium) was first discovered by British chemist Smithson Tennant. Osmium and iridium were identified in the black residue, which was left after dissolving platinum in aqua regia (a mixture of hydrochloric and nitric acids). These days, osmium is obtained either from mining nickel or platinum ores.
Metallic osmium is difficult to create. A powdered form of osmium is easier to make; however, it gives off a harmful and poisonous smell called osmium tetroxide (the word “osmium,” by the way, is from the Greek word osme, meaning “smell”). Because osmium smells particularly bad, it is mostly reserved for making alloys. You may find osmium in record player needles, ballpoint pen tips, fountain pen tips, and electrical contacts, among all other uses.
Iridium (Ir)
Osmium and iridium are found next right to each other in the periodic table, with the latter’s atomic number being 77. It has the symbol Ir.
British chemist Smithson Tennant discovered both iridium and osmium in 1803. Osmium and iridium were identified in the black residue, which was left after dissolving platinum in aqua regia (a mixture of hydrochloric and nitric acids). Nowadays, iridium is mostly obtained from the processing of nickel and platinum ores.
Iridium is considered one of the rarest elements on Earth; only about three tons of iridium are produced annually. In its purest form, iridium is very brittle and is nearly impossible to work with. Iridium is mostly used as a hardening agent for platinum, ending up together as an alloy. Platinum-iridium alloys are mostly used as crucibles and other equipment at a naturally high temperature. Iridium is also used as an alloy for osmium to create the tips of fountain pens and compass bearings.
Oganesson (Og)
Oganesson was temporarily named Ununoctium until 2016. It is a transactinide element (elements from 104 to 118 in the periodic table, meaning they are immediately greater than the actinides). It has the atomic number 118 in the periodic table and the symbol Og. The name literally means “one-one-eight.”
In 2006, scientists working at the Joint Institute for Nuclear Research in Dubna, Russia, and Lawrence Livermore National Laboratory in California, US, announced the creation of ununoctium by bombarding atoms of californium-249 with ions of calcium-48. This resulted in ununoctium-294, which is a stable isotope with a half-life of 0.89 milliseconds when calculated. It decays into livermorium-290 through alpha decay.
Ununoctium is a rare element since very little of it has been produced. It has no known practical uses outside of scientific research.
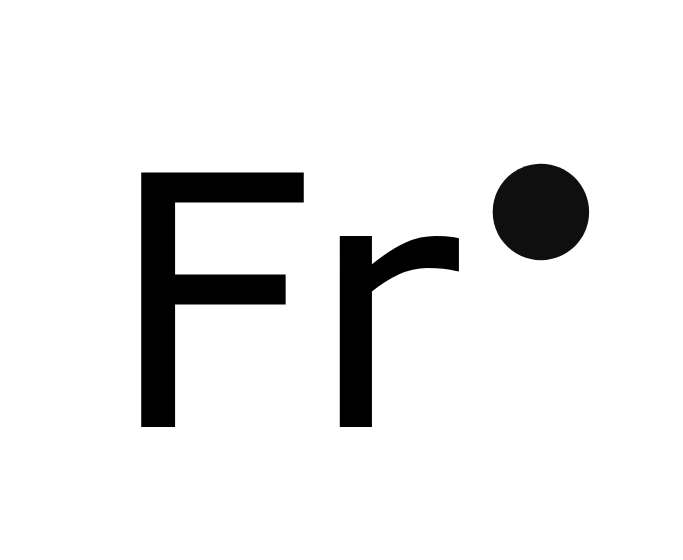
Francium (Fr)
Francium is a chemical element with the symbol Fr and atomic number 87. Although francium is a naturally occurring element, scientists have extracted no more than an ounce of it from the Earth’s crust at one time. Since naturally occurring francium is very rare, scientists produce it for scientific research purposes.
In 1939, French physicist Marguerite Perey discovered the presence of this element while she was analyzing actinium’s decay sequence. Francium can be produced by bombarding thorium with protons, deuterons, and helium ions or by bombarding radium with neutrons.
Francium-223 is francium’s most stable isotope. It has a half-life of about 22 minutes. A highly radioactive metal, it decays into astatine-219 through alpha decay, into radium-223 through beta decay, or into radon. Since it is very rare, it has no known commercial uses apart from its use in scientific research.
Technetium (Tc)
Technetium has the symbol Tc and atomic number 43. It is considered the first-ever predominantly artificially-produced element, first discovered by two Italian scientists, Emilio G. Segre and Carlo Perrier, in 1937. Technetium was developed by bombarding molybdenum atoms with deuterons that had been accelerated by a special device called a cyclotron.
Today, the production of technetium involves bombarding molybdenum-98 with neutrons. The neuron activation of molybdenum-98 leads into molybdenum-99. Molybdenum-99, whose half-life is 65.94 hours, decays into technetium-99 through beta decay.
Technetium-98 is technetium’s most stable isotope and has a half-life of about 4.2 million years. It decays into ruthenium-98 through beta decay.
Technetium, in small amounts, can slow down the corrosion of steel, although technetium’s high radioactivity poses problems that limit its application to closed systems. It is also used in equipment calibration and as a medical tracer.
Neptunium (Np)
Neptunium, with the atomic number 93, occupies a unique position in the periodic table as one of its rarest elements. Discovered in 1940 by Edwin McMillan and Philip H. Abelson, neptunium was the first transuranium element to be synthesized.
Named after the planet Neptune, it symbolizes the element’s place beyond uranium in the periodic table, as Neptune lies beyond Uranus in the solar system. This radioactive element is not found naturally in significant amounts on Earth and is primarily produced through the neutron bombardment of uranium in nuclear reactors.
The scarcity of neptunium is compounded by its half-life; its most stable isotope, neptunium-237, has a half-life of about 2.14 million years, contributing to its rarity and limiting its accumulation in nature. Neptunium-237’s unique properties have made it a subject of interest in scientific research, particularly in the study of nuclear fission reactions and potential uses in nuclear waste management.
Interesting Facts About the Periodic Table
Beyond its well-acknowledged scientific significance, the periodic table harbors a multitude of fascinating stories, groundbreaking discoveries, and intriguing facts. Here are ten interesting facts about the periodic table that highlight its complexity, history, and the curious tidbits that make it endlessly fascinating.
- Mendeleev’s Dream: Dmitri Mendeleev, the father of the periodic table, claimed that the idea of arranging the elements came to him in a dream. He woke up and immediately wrote down what would become the foundation of modern chemistry.
- Element Naming Controversies: The naming of elements has not always been straightforward, leading to disputes. For instance, element 106 was contested between American and Soviet scientists, eventually named Seaborgium (Sg) in honor of American chemist Glenn T. Seaborg.
- Gallium’s Spoon Trick: Discovered in 1875 by Lecoq de Boisbaudran, gallium has a melting point of approximately 29.76°C (85.57°F), allowing it to melt in your hand or when used to make a spoon dipped in hot tea.
- Only One Letter: The periodic table features only one element with a single-letter symbol: Hydrogen (H).
- Newest Members: As of my last update, the most recently added elements are Nihonium (Nh), Moscovium (Mc), Tennessine (Ts), and Oganesson (Og), completing the seventh row of the periodic table in 2016.
- Element 61, A Missing Piece: Promethium, element number 61, was one of the last naturally occurring elements to be discovered, filling a gap in the periodic table in 1945.
- The Noblest of Them All: Helium (He) is the only element that was first discovered outside of Earth, observed in the sun’s spectrum before it was found on our planet.
- Mercury’s Liquid Form: Among all the elements, only mercury (Hg) is liquid at room temperature, making it unique in its category.
- Artificially Created Elements: Elements beyond uranium (element 92) are not found in nature and have been synthesized in laboratories, showcasing human ingenuity in expanding the periodic table.
- Universe’s Building Blocks: Hydrogen and helium, the two simplest elements, make up about 99% of the known universe, highlighting the simplicity at the heart of cosmic complexity.
Conclusion
The scarcity of these rare elements challenges scientists and researchers to push the boundaries of detection, synthesis, and application, driving innovation and discovery. As we continue to probe the depths of the atomic world, the stories of these rare elements remind us of the endless quest for knowledge that defines humanity’s relationship with science.